June 25, 2024, Shanghai, China—Alebund Pharmaceuticals (“Alebund” or the “Company”), an integrated biopharmaceutical company focusing on developing innovative therapies for the treatment of renal diseases and related chronic conditions, announced the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) to AP306 for the treatment of hyperphosphatemia in patients with chronic kidney disease (CKD).
AP306 is the world’s first and currently the only pan-inhibitor of sodium-dependent phosphate transporters in clinical development. According to the official announcement by the CDE, the expert panel has considered AP306 as an innovative drug due to its novel mechanism of action. This drug has demonstrated clear clinical improvements over existing treatment. This designation is granted to AP306 in recognition of its compliance with the regulatory standards outlined in the Administrative Measures for Drug Registration and the Working Procedures for Review of Breakthrough Therapy Drugs (Interim), etc.
In the completed AP306 phase 2 clinical trial (CTR20230189; NCT05764590), AP306 monotherapy demonstrated a significant decrease in serum phosphate after treatment. There was a much higher proportion of patients who achieved the KDIGO guidelines recommended target range of serum phosphate (2.5 – 4.5 mg/dL) in the AP306 group compared with the active comparator group (sevelamer carbonate). In addition, a lower mean daily dose was observed in the AP306 group compared with the sevelamer group. The study results were recently presented at the 61st European Renal Association (ERA) Congress (link to the data presented at the ERA Congress).
“Hyperphosphatemia remains an important clinical problem in patients undergoing dialysis; its control rate has been suboptimal despite the widespread use of currently available phosphate-lowering drugs. Compared with existing treatment options, AP306 is expected to substantially improve serum phosphate control and become a new treatment choice for patients with hyperphosphatemia.” Dr. Jin Tian, Co-founder and CMO of Alebund commented.
“Receiving Breakthrough Therapy Designation is a significant regulatory milestone in developing Alebund’s innovative drugs for patients with chronic kidney disease,” stated Dr. Gavin Xia, Co-founder, Chairman, and CEO of Alebund. “We will continue to advance the development of AP306 in China and globally with full speed.”
The NMPA BTD is intended to facilitate and expedite developing and reviewing an investigational drug to treat a serious disease or condition for which there is no existing treatment and where preliminary clinical evidence indicates that the drug has demonstrated substantial improvement over currently available therapies. The BTD will qualify a drug candidate to receive status for rapid review by the CDE and allow the sponsor to obtain timely advice and communication from the CDE to accelerate the approval in addressing the clinical needs of patients.
About Hyperphosphatemia
Hyperphosphatemia is one of the most common complications in CKD patients. The long-term elevated serum phosphate level could cause multiple complications such as secondary hyperparathyroidism, renal osteodystrophy, and vascular calcification. It is an independent risk factor of cardiovascular events and all-cause mortalities. A good control of serum phosphate levels could effectively improve the patient’s outcome. For CKD patients undergoing dialysis treatment, regular dialysis is not sufficient to remove the overload of serum phosphate in the body. Considering the limitations of low-phosphate diet which might cause dystrophia, oral use of phosphate binders is the prevailing treatment for hyperphosphatemia. However, less than 50% can maintain reaching a good phosphate control with current treatment options.
About AP306
AP306 (EOS789) is an oral inhibitor of phosphate transporters, NaPi-IIb, PiT-1, PiT-2, which was discovered by Chugai Pharmaceutical Co., Ltd. Under the option and license agreement between Alebund and Chugai in 2021, Alebund conducted the phase 2 study with data reported in this release. Alebund has fully executed the global rights option and now owns the global development and commercialization rights for AP306.
About Alebund Pharmaceuticals
Alebund is a biopharmaceutical company jointly incubated by a group of industry leaders in the field of nephrology in Shanghai in 2018. Alebund focuses on the discovery and development of novel therapies primarily for kidney diseases and their complications, as well as other chronic conditions. Alebund has built a diversified and balanced pipeline of drug candidates targeting a range of renal diseases, including chronic kidney disease (CKD)/dialysis complications, IgA nephropathy, diabetic kidney disease, and autosomal dominant polycystic kidney disease (ADPKD). Alebund’s pipeline comprises both small-molecule and biological assets, in which the most advanced program is undergoing a pivotal phase 3 study.
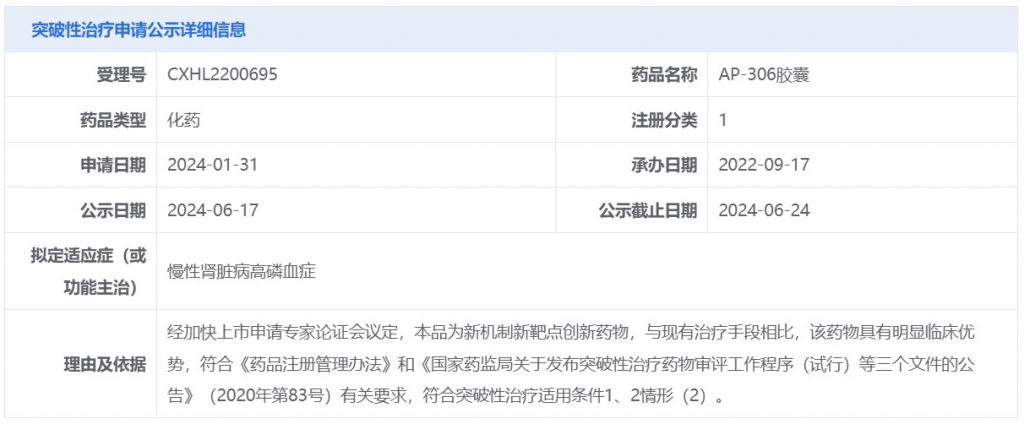
礼邦医药在研创新药物 AP306 获国家药品监督管理局突破性治疗药物认定用于治疗慢性肾脏病高磷血症
2024 年 6 月 25 日,中国上海—礼邦医药( “礼邦” 或“公司”)是一家专注于开发治疗肾脏疾病及其它相关慢性疾病创新药物的综合性生物制药公司,公司宣布其在研创新药物 AP306 获国家药品监督管理局药品审评中心突破性治疗药物认定用于治疗慢性肾脏病高磷血症。
AP306 是全球首创且目前唯一处于临床开发阶段的钠依赖性磷酸盐转运蛋白泛抑制剂。国家药品监督管理局药品审评中心此次突破性治疗药物认定主要考量包括 AP306 为新机制新靶点创新药物,与现有治疗手段相比,AP306 具有明显临床优势。

在 AP306 已完成的 Ⅱ 期临床试验(CTR20230189; NCT05764590)中,慢性肾脏病血液透析合并高磷血症患者在接受 AP306 单药治疗后血清磷水平显著下降。与阳性对照组(碳酸司维拉姆)相比,AP306 组达到 KDIGO 指南推荐的血清磷目标范围(2.5 – 4.5 mg/dL)的患者比例明显高于碳酸司维拉姆组。此外,AP306 组的平均每日剂量也远低于碳酸司维拉姆组。这项临床试验的研究结果已在近期召开的第 61 届欧洲肾脏协会(ERA)年会上公布(点击查看在ERA年会上公布的数据)。
“对于透析患者而言,高磷血症的治疗仍然是一个重要的临床问题。虽然目前磷酸盐结合剂已被广泛运用,但患者血磷达标率极不理想。同现有治疗药物相比,AP306 有望大幅提升血磷控制,为高磷血症患者提供新的治疗选择。” 礼邦医药联合创始人兼首席医学官田劲医生说道。
“礼邦医药致力于开发慢性肾脏病相关创新药物,此次获得的突破性治疗药物认定是 AP306 研发道路上一个重要的里程碑。” 礼邦联合创始人、董事长兼首席执行官夏国尧博士评论道,“我们将继续在国内外全速推进 AP306 的临床开发进程。”
国家药品监督管理局突破性治疗药物程序旨在推动和加速药物开发和审批流程,主要针对防治严重危及生命或者严重影响生存质量的疾病且尚无有效治疗手段或者与现有治疗手段相比有足够证据表明具有明显临床优势的创新药。获得突破性治疗药物认定的可以获得药审中心优先配置资源进行沟通交流和进行上市许可的快速审评审批。
关于高磷血症
高磷血症是慢性肾脏病患者的最常见的并发症之一。血磷水平长期升高可导致甲状旁腺功能亢进、肾性骨病、血管钙化等多种并发症,是患者心血管事件和全因死亡的独立危险因素。控制血磷水平达标可有效改善慢性肾脏病患者预后。对于接受透析治疗的慢性肾脏病患者,即使规律透析也无法清除体内蓄积的磷酸盐。由于低磷饮食导致营养不良的限制,口服磷酸盐结合剂是目前高磷血症的主要治疗方法,但超过一半的患者通过现有的治疗选择仍不能有效的控制血磷。
关于 AP306
AP306(EOS789)是一种口服磷酸盐转运蛋白 NaPi-IIb、PiT-1、PiT-2 泛抑制剂,是全球首个且目前唯一进入临床开发阶段的磷酸盐转运蛋白泛抑制剂,由日本中外制药有限公司发现。根据礼邦与中外制药 2021 年签订的选择权和许可协议,礼邦完成了本新闻稿中提及的 Ⅱ 期临床研究。礼邦已全面行使选择权,目前独家拥有 AP306 的全球开发和商业化权利。
关于礼邦医药
2018 年初,礼邦医药由顶尖的肾脏病领域行业领导者孵化于中国上海。作为一家生物制药公司,礼邦主要致力于肾脏病以及其相关慢性疾病创新药物的发现和开发,为慢性肾脏病及相关疾病患者提供更佳临床治疗方案。礼邦医药已经建立起了丰富且均衡的肾脏病新药产品管线,包括针对慢性肾病(CKD)/透析并发症、IgA 肾病、糖尿病肾病、常染色体显性多囊肾病(ADPKD)等产品,公司在研产品包括小分子药物和生物制剂,进展最快的项目正在进行关键 Ⅲ 期临床试验。