- In the completed phase 2 study (NCT05764590), AP306 monotherapy significantly reduced serum phosphate and substantially improved the serum phosphate control rate in hemodialysis patients with hyperphosphatemia. AP306 was safe and well-tolerated.
- Alebund is actively advancing further clinical development of AP306.
May 27, 2024, Shanghai, China—Alebund Pharmaceuticals (“Alebund” or the “Company”), an integrated biopharmaceutical company focusing on developing innovative therapies for the treatment of renal diseases and related chronic conditions, presented the phase 2 study results of AP306 in hemodialysis patients with hyperphosphatemia through the Focused Oral at the 61st European Renal Association (ERA) Congress in Stockholm, Sweden.
AP306 is a first-in-class, pan-inhibitor of sodium-dependent phosphate transporters, which blocks the active phosphate transport in the intestine and has the potential to overcome limitations of dietary phosphate restriction and phosphate binders. The phase 2 study is a randomized, open-label, active-controlled study; it involved 11 investigational sites in China and was led by Dr. Li Wang from Sichuan Provincial People’s Hospital.
In this phase 2 study,
- 55 patients were randomized and received AP306 (N=27) and sevelamer carbonate (N=28) for 12 weeks. The dose of AP306 and sevelamer was adjusted every 4 weeks to keep serum phosphate in the target range of 3.5 to 5.5 mg/dL. The investigational dose of AP306 was initiated at 75 mg and increased stepwise to 125 mg, and 150 mg three times a day with meals.
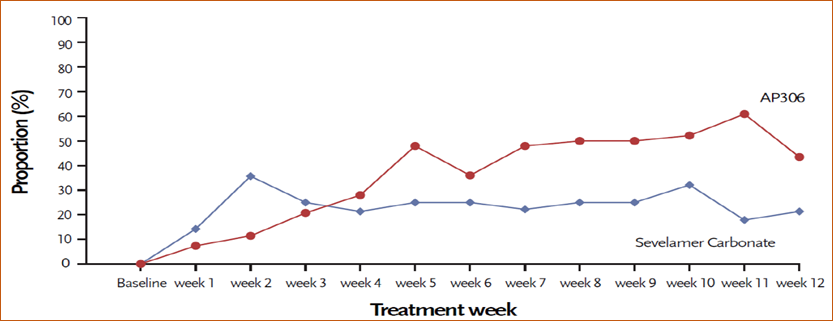
- The primary efficacy endpoint was defined as the mean changes in serum phosphate from baseline to the end of treatment. Both groups achieved a statistically significant decrease in the primary efficacy endpoint, which was -2.51 mg/dL (95% confidence interval: -3.07, -1.92) and -1.08 mg/dL (95% confidence interval: -1.58, -0.59) in the AP306 and sevelamer groups respectively (see Table 1).
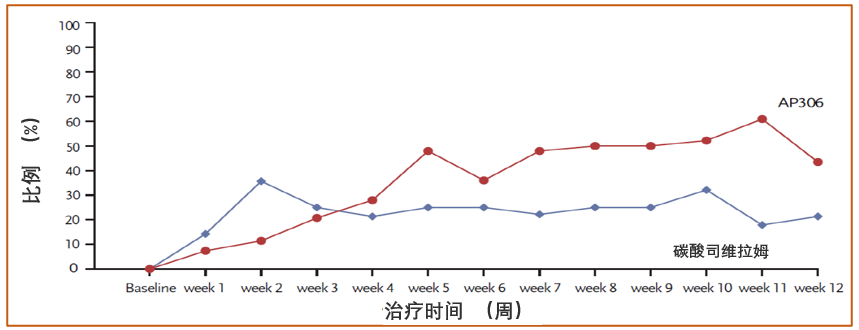
- The mean serum phosphate level gradually declined after AP306 initiation till the end of the treatment. The AP306 group achieved lower mean serum phosphate compared to the sevelamer group starting from the end of week 3 (see Figure 1). The proportion of patients who achieved the study-defined target range of serum phosphate (3.5 – 5.5 mg/dL) was comparable between the two groups, while a much higher proportion of patients achieved KDIGO recommended target range of serum phosphate (2.5 – 4.5 mg/dL) in the AP306 group compared with the sevelamer group (see Figure 2).
- A lower mean daily dose was observed in the AP306 group compared with the sevelamer group during the last 4 weeks of treatment, which were 288.30 mg/day and 4650.99 mg/day respectively.
- The most common adverse events in the AP306 group were gastrointestinal disorders (51.9%) and mainly diarrhea (44.4%). All the diarrhea events were Grade 1/2 and mostly resolved within 2 weeks. No serious adverse event was reported in the AP306 group.
“Data from this proof-of-concept study of AP306 are encouraging and promising for substantially improving phosphate control in dialysis patients with hyperphosphatemia,” said Dr. Jin Tian, Co-founder and CMO of Alebund. Alebund will advance the clinical development for AP306.
61st European Renal Association Congress Focussed Orals Presentation Details
Date: Friday, May 24, 2024
Title: A Phase 2 Trial to Evaluate Serum Phosphate Lowering Effect of a Pan-phosphate Transporter Inhibitor AP306 in Dialysis Patients with Hyperphosphatemia
Table 1 Primary Efficacy Endpoint: Change in serum phosphate from baseline to end of treatment (EOT)
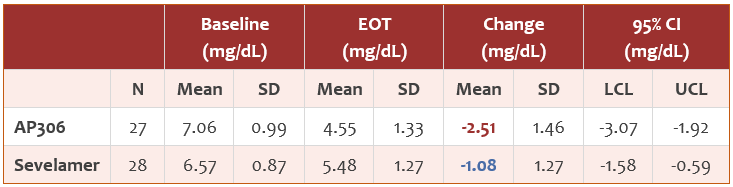
Figure 1 Change in serum phosphate over the study
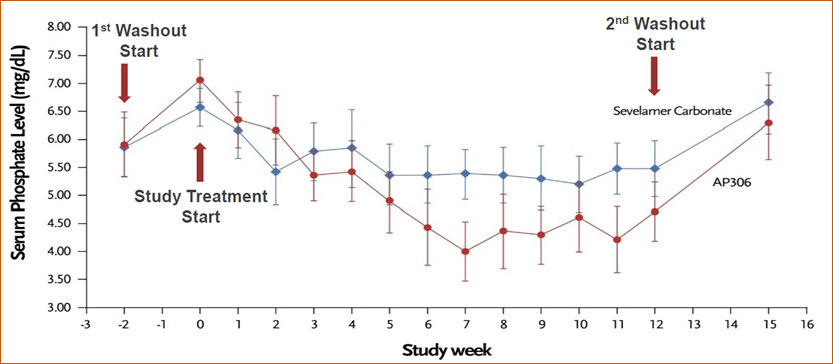
Figure 2 Proportion of patients with serum phosphate 2.5 – 4.5 mg/dL
About Hyperphosphatemia
Hyperphosphatemia is one of the most common complications in CKD patients. The long-term elevated serum phosphate level could cause multiple complications such as secondary hyperparathyroidism, renal osteodystrophy, and vascular calcification. It is an independent risk factor of cardiovascular events and all-cause mortalities. A good control of serum phosphate levels could effectively improve the patient’s outcome. For CKD patients undergoing dialysis treatment, the regular dialysis is not sufficient to remove the overload of serum phosphate in the body. Considering the limitations of low-phosphate diet which might cause dystrophia, oral use of phosphate binders is the prevailing treatment for hyperphosphatemia. However, less than 50% can maintain reaching a good phosphate control with current treatment options.
About AP306
AP306 (EOS789) is an oral inhibitor of phosphate transporters, NaPi-IIb, PiT-1, PiT-2, which was discovered by Chugai Pharmaceutical Co., Ltd. Under the option and license agreement between Alebund and Chugai in 2021, Alebund conducted the phase II study with data reported in this release. Alebund has fully executed the global rights option and now owns the global development and commercialization rights for AP306.
About the Study
NCT05764590 is a phase 2 randomized, open-label, active-controlled, multicenter study to evaluate the safety and serum phosphate lowering effect of AP306 in chronic kidney disease patients receiving maintenance hemodialysis with hyperphosphatemia. The primary efficacy endpoint measures the change in serum phosphate level from the baseline to the end of the 12-week treatment or before the initiation of rescue therapy. Participants enrolled in the study were randomized in a 1:1 ratio to be treated with either AP306 or sevelamer carbonate.
About Alebund Pharmaceuticals
Alebund is a biopharmaceutical company jointly incubated by a group of industry leaders in the field of nephrology in Shanghai in 2018. Alebund focuses on the discovery and development of novel therapies primarily for kidney diseases and their complications, as well as other chronic conditions. Alebund has built a diversified and balanced pipeline of drug candidates targeting a range of renal diseases, including chronic kidney disease (CKD)/dialysis complications, IgA nephropathy, diabetic kidney disease, and autosomal dominant polycystic kidney disease (ADPKD). Alebund’s pipeline comprises both small-molecule and biological assets, in which the most advanced program is undergoing a pivotal phase III study.
礼邦医药在第 61 届欧洲肾脏协会(ERA)年会上公布其 AP306 Ⅱ期临床试验数据
- 在已完成的Ⅱ期临床研究(NCT05764590)中,AP306 单药治疗显著降低血液透析合并高磷血症患者的血清磷,并大幅提高血清磷控制率。AP306 被证实安全且耐受良好。
- 礼邦医药正在积极推进 AP306 的进一步临床开发。
2024 年 5 月 27 日,中国上海—礼邦医药( “礼邦” 或“公司”)是一家专注于开发治疗肾脏疾病及其相关慢性疾病创新药物的综合性生物制药公司,公司宣布于当地时间 5 月 24 日在瑞典斯德哥尔摩举行的第 61 届欧洲肾脏协会(ERA)年会上以专题口头报告 (Focused Oral)形式公布其 AP306 在血液透析合并高磷血症患者中的Ⅱ期临床研究结果。
AP306 是一种首创的钠依赖性磷酸盐转运蛋白泛抑制剂,能阻断肠道磷酸盐主动转运,有望克服低磷饮食和磷结合剂治疗高磷血症的局限性。此次汇报的研究是一项随机、开放标签、阳性药物对照的Ⅱ期临床试验,由四川省人民医院王莉教授牵头,在中国 11 家研究中心完成。
在这项Ⅱ期临床研究中:
- 55 例血液透析合并高磷血症患者随机接受 AP306(N=27)和碳酸司维拉姆(N=28)治疗 12 周。每 4 周调整一次 AP306 和碳酸司维拉姆的剂量,以保持血清磷控制在 3.5–5.5 mg/dL 的目标范围。AP306 的试验剂量从每次 75 mg、一日三次开始,随餐服用,逐步增加到每次 125 mg 和 150 mg、一日三次。
- 两组在主要疗效终点,即从基线到治疗结束时血清磷平均值的变化,均达到有统计学意义的下降,分别为 AP306 组-2.51 mg/dL(95%置信区间:-3.07,-1.92),碳酸司维拉姆组-1.08 mg/dL(95%置信区间:-1.58,-0.59)(见表 1),AP306 组血清磷下降幅度明显高于碳酸司维拉姆组。
- 在治疗期间,AP306 组血清磷平均值随治疗的延长而逐渐降低,从第 3 周末开始低于碳酸司维拉姆组并持续至治疗结束(见图 1)。虽然达到研究定义的血清磷目标范围(3.5 – 5.5 mg/dL)的患者比例在两组之间相当,但 AP306 组达到 KDIGO 推荐的血清磷目标范围(2.5 – 4.5 mg/dL)的患者比例明显高于碳酸司维拉姆组(见图 2)。
- 在治疗的最后 4 周,AP306 组的平均每日剂量低于碳酸司维拉姆组,分别为 288.30 mg/天和 4650.99 mg/天。
- AP306 组最常见的不良事件为胃肠道反应(51.9%),主要是腹泻(44.4%)。所有腹泻事件严重程度均为 1/2 级且大多数在 2 周内自行缓解。AP306 组未报告严重不良事件。
礼邦医药联合创始人兼首席医学官田劲医生表示:“AP306 概念验证研究的数据令人鼓舞,AP306 治疗有望显著改善透析合并高磷血症患者的血磷控制情况。”礼邦将继续推进 AP306 在全球的临床开发。
第 61 届欧洲肾脏协会大会报告信息
时间:2024 年 5 月 24 日星期五
标题:一项评估磷酸盐转运蛋白泛抑制剂 AP306 在透析合并高磷血症患者中降低血清磷作用的Ⅱ期试验
表 1 主要疗效终点:血清磷从基线到治疗结束时的变化
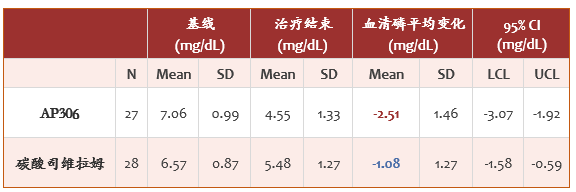
图 1 研究期间血清磷随时间变化
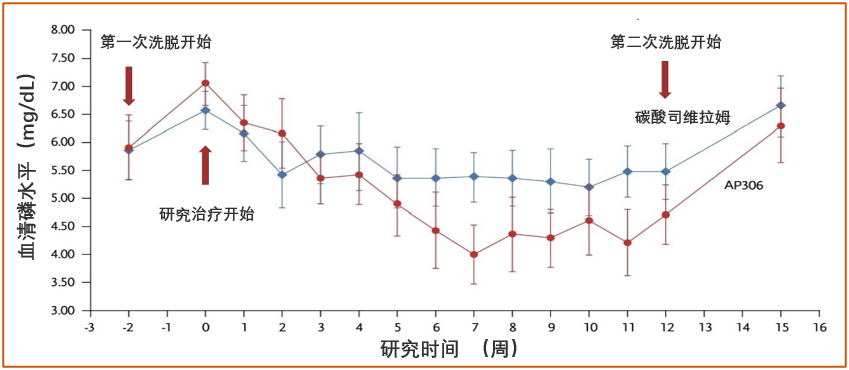
图 2 血清磷在 2.5 – 4.5 mg/dL 之间的患者比例
关于高磷血症
高磷血症是慢性肾脏病患者的最常见的并发症之一。血磷水平长期升高可导致甲状旁腺功能亢进、肾性骨病、血管钙化等多种并发症,是患者心血管事件和全因死亡的独立危险因素。控制血磷水平达标可有效改善慢性肾脏病患者预后。对于接受透析治疗的慢性肾脏病患者,即使规律透析也无法清除体内蓄积的磷酸盐。由于低磷饮食导致营养不良的限制,口服磷酸盐结合剂是目前高磷血症的主要治疗方法,但超过一半的患者通过现有的治疗选择仍不能有效的控制血磷。
关于 AP306
AP306(EOS789)是一种口服磷酸盐转运蛋白 NaPi-IIb、PiT-1、PiT-2 泛抑制剂,是全球首个且目前唯一进入临床开发阶段的磷酸盐转运蛋白泛抑制剂,由日本中外制药有限公司发现。根据礼邦与中外制药 2021 年签订的选择权和许可协议,礼邦完成了本新闻稿中提及的 Ⅱ 期临床研究。礼邦已全面行使选择权,目前独家拥有 AP306 的全球开发和商业化权利。
关于此项研究
NCT05764590 是一项Ⅱ期随机、开放标签、阳性药物对照、多中心研究,旨在评估 AP306 在维持性血液透析合并高磷血症慢性肾脏病患者中的安全性和降血磷作用。研究的主要疗效终点是评估血清磷水平从基线到 12 周治疗结束或开始补救治疗之前的变化。该研究参与者以 1:1 比例随机接受 AP306 或碳酸司维拉姆治疗。
关于礼邦医药
2018 年初,礼邦医药由顶尖的肾脏病领域行业领导者孵化于中国上海。作为一家生物制药公司,礼邦主要致力于肾脏病以及其相关慢性疾病创新药物的发现和开发,为慢性肾脏病及相关疾病患者提供更佳临床治疗方案。礼邦医药已经建立起了丰富且均衡的肾脏病新药产品管线,包括针对慢性肾病(CKD)/透析并发症、IgA 肾病、糖尿病肾病、常染色体显性多囊肾病(ADPKD)等产品,公司在研产品包括小分子药物和生物制剂,进展最快的项目正在进行关键Ⅲ期临床试验。