- Efficacy: Clinically and statistically significant superiority of AP301 maintenance dose versus ineffective low dose in reducing serum phosphate levels was established and AP301’s efficacy is sustained throughout the 52 weeks of treatment. The non-inferiority of AP301 to sevelamer carbonate in reducing serum phosphate levels was demonstrated.
- Safety: AP301 was safe and well-tolerated. The most common AEs were discolored feces and diarrhea. Diarrhea generally occurred early and resolved without treatment changes. No evidence of iron accumulation was observed in the study.
November 7, 2025, Shanghai, China — Alebund Pharmaceuticals (“Alebund” or the “Company”), an integrated biopharmaceutical company focusing on developing innovative therapies for the treatment of renal diseases and related chronic conditions, presented the pivotal phase 3 study results of AP301 in patients on hemodialysis and peritoneal dialysis with hyperphosphatemia (RESPOND-1 study, NCT07030595) at the American Society of Nephrology (ASN) 2025 Congress in Houston, Texas.
AP301 is a novel fiber-iron-based phosphate binder that offers high phosphate-binding capability, does not require chewing before swallowing, does not expand in volume when exposed to gastric fluid, and is not systemically absorbed. These features contribute to a reduced pill burden, improved tolerability, and enhanced patient adherence.
The RESPOND-1 study was a randomized, open-label, active-controlled, multi-center study designed to evaluate the efficacy and safety of AP301 in controlling serum phosphate levels in dialysis patients with hyperphosphatemia. The 52-week study included an active control phase, an AP301 low-dose control phase, and an extended treatment phase. Sevelamer carbonate served as the active comparator throughout the entire study period. The study was conducted at 50 investigational sites in China, and was led by Professor Li ZUO, Director of the Department of Nephrology at Peking University People’s Hospital.
In this study:
- Of 692 participants screened, 474 participants were randomized (3:1) to the AP301 or sevelamer carbonate arm; 187 eligible participants in AP301 arm were further re-randomized (1:1) at Week 24 to the AP301 maintenance dose (N=94) or low dose (N=93) arm. Overall, 396 (84%) participants completed the study.
- The primary efficacy endpoints included: 1) the change in serum phosphate levels during the low dose control phase from Week 24 to Week 27 between the AP301 maintenance dose and low dose arm in responders defined as serum phosphate level <1.78 mmol/L (5.5 mg/dL) by Week 20; 2) the change in serum phosphate levels from baseline to Week 12 between AP301 and sevelamer carbonate during active control phase.
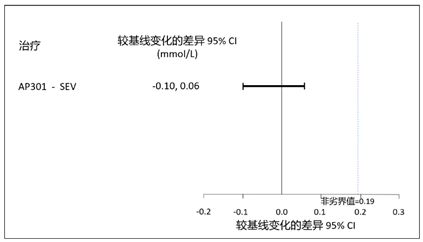
- At week 12, the least squares mean (LSM) reduction from baseline was 0.72 mmol/L (2.22 mg/dL) for AP301, compared to 0.70 mmol/L (2.17 mg/dL) for sevelamer carbonate. AP301 demonstrated non-inferiority to sevelamer carbonate, with an LSM difference of -0.02 mmol/L(95% confidence interval (CI): -0.10, 0.06)[-0.06 mg/dL (95% CI: -0.31, 0.20)]; the upper CI bound of 0.06 mmol/L (0.20 mg/dL) was below the pre-defined non-inferiority margin (NIM) of 0.19 mmol/L (0.59 mg/dL). (see Figure 1)
- At week 27, AP301 maintenance dose showed a clinically and statistically significant superiority on serum phosphate control over an ineffective AP301 low dose in the low dose control phase {LSM difference -0.58 mmol/L (95% CI: -0.69, -0.47; P <0.001) [-1.8 mg/dL (95% CI: –2.1, –1.5; P <0.001)]}. (see Figure 2)
- AP301 achieved robust and sustained serum phosphate reduction over 52 weeks, suggesting its long-term therapeutic benefit (See Figure 3). It also showed a numerically higher serum phosphate response rate in the AP301 arm (66.7%) compared to the sevelamer carbonate arm (58.6%) at Week 52, and with a lower mean daily dose exposure (6.52 g/day in AP301 versus 7.56 g/day in sevelamer carbonate).
- Most participants experienced at least one AE (96.3% in AP301 and 90.8% in sevelamer carbonate). Diarrhea was the most common AE leading to study discontinuation in the AP301 arm (2/355, 0.6%), typically occurring within the first 2–4 weeks and predominantly mild in severity.
- Iron parameters changed from baseline mainly during the first 24 weeks, then stabilized or decelerated thereafter. No evidence of iron accumulation was observed with cumulative AP301 exposure.
Presentation Details at The American Society of Nephrology (ASN) 2025 Congress
Date: November 6, 2025
Title: 52-Week Phase 3 Study to Evaluate the Efficacy and Safety of a Novel Iron-Based Phosphate Binder AP301 in Patients on Dialysis with Hyperphosphatemia
Figure 1 Difference of Change in Serum Phosphate from Baseline to Week 12

Figure 2 Change of Serum Phosphate during LD Phase (Week 24 to Week 27)*
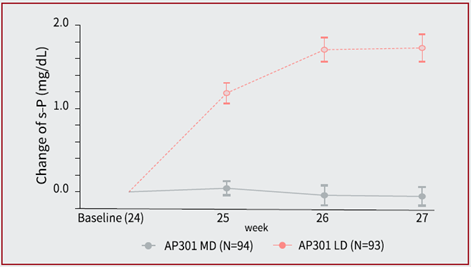
* Data are presented as mean±SE
Figure 3 Change of Serum Phosphate over Time (Baseline to Week 52)*
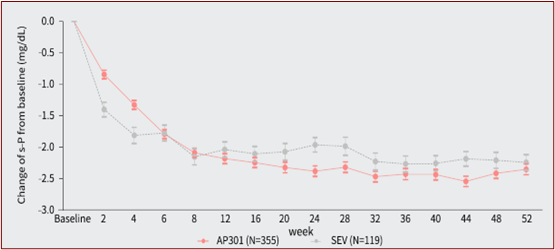
* Data from LD phase was excluded; Data are presented as mean±SE
“Iron-based phosphate binders have been used with increasing frequency in recent years for certain advantages. As a new generation of iron-based phosphate binder, AP301 is well differentiated from other iron-based phosphate binders with no systemic absorption and no need to chew or crush before swallowing. This pivotal trial demonstrates the clinical value of AP301 and its potential to advance the treatment option for patients with hyperphosphatemia.” Dr. Jin Tian, Co-founder and Chief Medical Officer of Alebund, commented, “Alebund is actively engaging in discussions with the China National Medical Products Administration regarding the new drug application plan for AP301.”
About Hyperphosphatemia
Hyperphosphatemia is one of the most common complications in CKD patients. The long-term elevated serum phosphate levels could cause multiple complications such as secondary hyperparathyroidism, renal osteodystrophy, and vascular calcification. It is an independent risk factor of cardiovascular events and all-cause mortalities. Good control of serum phosphate levels could effectively improve the patients’ outcome. For CKD patients undergoing dialysis treatment, regular dialysis is not sufficient to remove the excess serum phosphate in the body. Considering the limitations of low-phosphate diet which might cause dystrophia, oral use of phosphate binders is the prevailing treatment for hyperphosphatemia. However, existing phosphate binders lead to low patient compliance, with less than 50% of patients achieving good phosphate control, due to gastrointestinal side effects and high pill burden, etc.
According to the Global and Chinese Hyperphosphatemia Drug Industry Blue Book by China Insights Consultancy in 2023, the out-of-target rate of serum phosphate level in dialysis patients in Chinese mainland was significantly higher than that in other countries and regions. There remains substantial room for improvement in terms of the proportion of patients using phosphate binders and duration of their usage. The market size of serum phosphorus-lowering products in China is expected to reach RMB10 billion by 2035.
About Alebund Pharmaceuticals
Alebund was founded in Shanghai in 2018. The Company focuses on the discovery, development, manufacturing, and commercialization of novel therapies primarily for kidney diseases and their complications, as well as other chronic conditions, to bring better therapeutic options to patients in China and globally. Alebund has built a diversified and balanced pipeline of seven drug candidates and one commercialized product (Mircera®) targeting a broad range of renal indications, including chronic kidney disease (CKD) and CKD complications, such as hyperphosphatemia, renal anemia, IgA nephropathy, diabetic kidney disease, focal segmental glomerulosclerosis (FSGS), and autosomal dominant polycystic kidney disease (ADPKD). Alebund has completed the construction of its manufacturing site in Yangzhou that will supply both drug substance and drug products of our product candidates, including AP301, upon commercial launch. Alebund has also established a dedicated in-house commercialization team in China responsible for promoting our renal products.
礼邦医药在 2025 年美国肾脏病年会上公布其磷结合剂 AP301 的 Ⅲ 期临床试验数据
- 有效性:AP301 维持剂量在降低血清磷水平上,相较于无效低剂量具有临床及统计学上的显著优效性,且在整个 52 周的治疗期内,AP301 的疗效稳健且持久,达到试验设计的降低血清磷水平方面不劣于碳酸司维拉姆的终点。
- 安全性:AP301 安全且耐受性良好。最常见的不良事件是粪便变色和腹泻。腹泻通常发生于治疗早期,无需调整治疗方案即可缓解;且研究中未观察到明显的铁过载风险信号。
2025 年 11 月 7 日,礼邦医药(“礼邦”或“公司”),一家专注于开发治疗肾脏疾病及相关慢性病创新药物的综合性生物制药公司,宣布公司在美国德克萨斯州休斯顿举行的 2025 年美国肾脏病年会(ASN)上公布其在研新型口服含铁磷结合剂 AP301 胶囊用于治疗透析(包括血液透析和腹膜透析)患者高磷血症的关键 Ⅲ 期临床研究(RESPONDER-1 研究,NCT07030595,CTR20231624)结果。
作为一款以纤维-铁为基础的新一代口服磷结合剂,AP301 有非常高的磷结合能力,无需咀嚼,在胃液中膨胀体积小,无系统吸收等优势。这些特点有助于减少患者每日服药数量,带来更好的安全性及胃肠耐受性并提升患者依从性。
本项关键 Ⅲ 期研究是一项随机、开放标签、阳性对照、多中心临床试验,旨在评估 AP301 对高磷血症透析患者血清磷控制的有效性和安全性。 整个研究治疗为期 52 周,包括阳性药物对照期,低剂量对照期和延长治疗期。碳酸司维拉姆作为阳性对照药物,在整个研究期间持续给药。该项研究在中国 50 家研究中心开展,共随机入组了 474 名参与者。北京大学人民医院肾内科主任、博士生导师左力教授担任该研究的牵头研究者。
在这项研究中:
- 在筛选的 692 名参与者中,474 名被随机(3:1)分配至 AP301 组或碳酸司维拉姆组;在第 24 周,187 名符合条件的 AP301 组参与者按 1:1 再随机分配至 AP301 维持剂量(N=94)或无效低剂量(N=93)组。396 名(84%)参与者完成了研究。
- 研究主要疗效终点包括:1) 对 AP301 治疗产生应答(定义为第 20 周末血清磷水平 <1.78 mmol/L [5.5 mg/dL])参与者继续使用 AP301 维持剂量组与 AP301 低剂量对照组之间从第 24 周末至第 27 周末或低剂量对照期治疗结束(以先发生者为准)的血清磷水平变化;2) AP301 组与碳酸司维拉姆组从基线至第 12 周末或治疗结束(以先发生者为准)的血清磷水平变化。
- 治疗至第 12 周,AP301 在降低血清磷水平方面非劣于碳酸司维拉姆。AP301 相比基线降幅最小二乘均值(LSM)为 0.72 mmol/L (2.22 mg/dL),碳酸司维拉姆降幅为 0.70 mmol/L (2.17 mg/dL),两者 LSM 差异为 -0.02 mmol/L (95% CI: -0.10, 0.06) [-0.06 mg/dL (95% CI: -0.31, 0.20)],其 95% CI 上限为 0.06 mmol/L (0.20 mg/dL),低于预设非劣界值 0.19 mmol/L (0.59 mg/dL)。(见图一)
- 治疗至第 27 周,AP301 维持剂量对比低剂量在降低血清磷水平上显示出具有临床及统计学上的显著优效性 {LSM 差异为 -0.58 mmol/L (95% CI: -0.69, -0.47; P <0.001) [-1.8 mg/dL (95% CI: –2.1, –1.5; P <0.001)]}。(见图二)
- AP301 在 52 周的治疗期内展现了稳健且持久的降磷效果,表明其能带来长期治疗获益。(见图三)此外,在 52 周时,AP301 组的血清磷达标率为 66.7%,高于碳酸司维拉姆组的 58.6%,且其平均剂量低于碳酸司维拉姆(6.52 克/天 vs. 7.56 克/天)。
- 大多数参与者至少经历过一次 AE(AP301 组 96.3%,碳酸司维拉姆组 90.8%)。腹泻是导致 AP301 组研究终止的最常见 AE(2/355, 0.6%),同时腹泻通常发生在治疗的前 2-4 周内,严重程度多为轻度。
- AP301 组参与者的铁参数相对于基线的变化主要发生在前 24 周,之后即保持平稳或变化趋缓。随着 AP301 暴露量的累积,未观察到明显的铁过载风险信号。
2025 年美国肾脏病年会报告信息
时间:2025 年 11 月 6 日 星期四
标题:一项为期 52 周在接受维持性透析的高磷血症患者中评价新型含铁磷结合剂 AP301 有效性和安全性的 III 期研究
图一 12 周时血清磷较基线变化的差异(mmol/L)
图二 第 24 至 27 周低剂量对照期内血清磷的变化(mmol/L)*
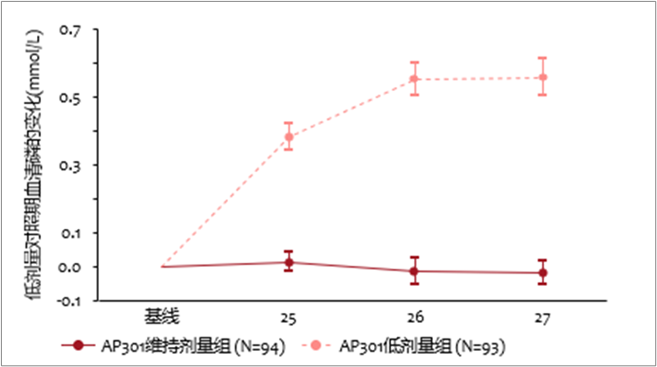
* 数据表示为平均值 ±SE。
图三 52 周治疗期内血清磷较基线的变化(mmol/L)*
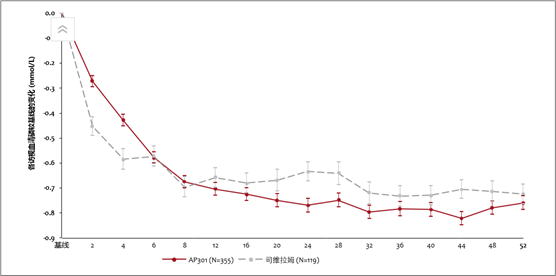
* 不包括低剂量对照期数据;数据表示为平均值(±SE)。
“近年来,含铁磷结合剂因其优势在临床中的使用日益广泛。作为新一代铁基磷酸盐结合剂,AP301 与其他铁基磷酸盐结合剂有显著不同。它不被全身吸收,亦无需咀嚼或压碎后吞咽。该项 Ⅲ 期临床研究进一步验证了 AP301 的临床价值。我们相信,作为新一代磷结合剂的 AP301 有望进一步提升患者依从性,为数百万高磷血症患者提供更优的治疗选择。“礼邦医药联合创始人、首席医学官田劲医生表示。“礼邦医药正积极就 AP301 产品的上市申请与中国国家药品监督管理局开展沟通。”
关于高磷血症
高磷血症是慢性肾脏病患者的重要并发症之一。血磷水平长期过高可导致甲状旁腺功能亢进、肾性骨病、血管钙化等多种并发症,是增加患者心血管事件和全因死亡的独立危险因素。控制血磷水平达标可有效改善慢性肾脏病患者的预后。对于慢性肾脏病接受透析治疗的高磷血症患者,即使规律透析也无法清除每日摄入磷酸盐在体内的蓄积量。由于饮食限磷的作用有限、且会影响患者的营养状况,口服磷结合剂是目前治疗高磷血症的主要方法, 但超过一半的患者血磷控制不佳,其中一个主要原因是现有磷结合剂的胃肠道副作用明显且服用药片数量过多,导致患者治疗依从性差。
根据灼识咨询 2023 年《全球及中国高磷血症药物行业蓝皮书》,中国在透患者血磷水平不达标率显著高于其他国家及地区,磷结合剂使用比例以及磷结合剂使用患者的用药时长方面均仍有较高提升空间。随着新一代降磷产品的上市,预计 2035 年中国降磷药物市场规模将达到百亿元人民币规模。
关于礼邦医药
2018 年初,礼邦医药创建于中国上海。作为一家生物制药公司,礼邦主要致力于肾脏病及其相关慢性疾病创新药物的发现,开发,生产和商业化,为全球慢性肾脏病及相关疾病患者提供更佳临床治疗方案。礼邦医药已经建立起了丰富且均衡的肾脏病新药产品管线,包括七个在研药物及一个已上市产品(美信罗®)。公司产品管线包括针对慢性肾病(CKD)及其并发症,如高磷血症,肾性贫血、IgA 肾病、糖尿病肾病、局灶阶段性肾小球硬化(FSGS)、常染色体显性多囊肾病(ADPKD)等的产品。礼邦医药已在扬州建成并启用药物生产基地,以支持礼邦管线产品包括 AP301 未来的商业化。同时,礼邦亦搭建了肾科专科销售团队负责相关产品的中国商业化推广。